Please be advised that Centennial Spine and Pain does NOT write prescriptions for an initial consultation.
Please be advised that Centennial Spine and Pain does NOT write prescriptions for an initial consultation.
The Intracept ® is an intraosseous nerve ablation procedure used in conjunction with radiofrequency (RF) generators for the ablation of basivertebral nerves for the relief of chronic low back pain that has not responded to at least six months of conservative care.
The Intracept ®Procedure is a minimally invasive procedure that targets the basivertebral nerve for the relief of chronic vertebrogenic low back pain.
Under fluoroscopic guidance, the Intracept Introducer Cannula is advanced through the pedicle.
The Intracept Curved Cannula is utilized to create a channel to the trunk of the basivertebral nerve.
The Intracept Radiofrequency Probe is inserted into the curved path and placed at the basivertebral nerve.
The Relievant Radiofrequency Generator is utilized to ablate the basivertebral nerve.


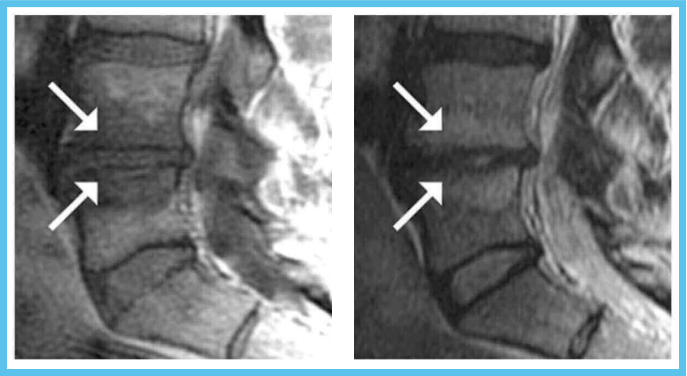
Hypointense T1W and Hyperintense T2W MR
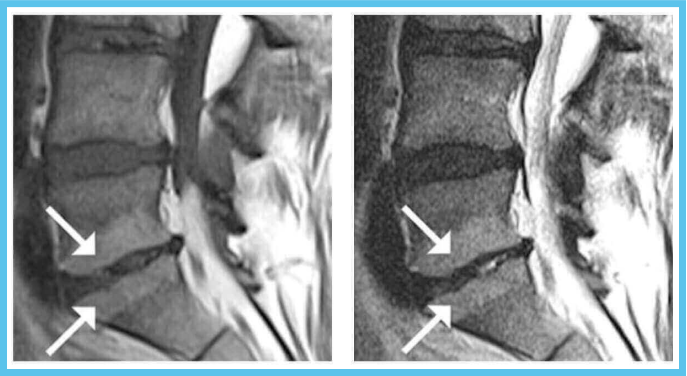
Hypointense T1W and Hyperintense T2W MR

The Intracept Intraosseous Nerve Ablation System is used in conjunction with radiofrequency (RF) generators for the ablation of basivertebral nerves of the L3 through S1 vertebrae for the relief of chronic low back pain.
The Intracept relieves chronic low back pain that is present for a period of at least six months, has not improved with at least six months of conservative care and a MRI that displays features consistent with Type 1 or Type 2 Modic changes and/or inflammation, edema, vertebral end plate changes, disruption and fissuring of the endplate, vascularized fibrous tissues within the adjacent marrow, hypointensive signals (Type 1 Modic change), and changes to the vertebral body marrow including, replacement of normal bone marrow by fat, and hyperintensive signals (Type 2 Modic change).

The Intracept Procedure is supported by two Level l randomized clinical trials. Intracept has demonstrated statistical significance compared to both standard care and sham, consistent improvement in both pain and function across both trials, and durable results beyond five years post-procedure. For more information click here.
